SUSTech Hong Chen’s team makes significant progress in the development of BDI electrodes
Jun 04, 2021
Recently, Associate Professor Hong Chen’s team from the School of Environmental Science and Engineering at the Southern University of Science and Technology (SUSTech) published their results on the future development of BDI electrodes, which paves the way for the energy-efficient BDI technique. Their paper, entitled “Electrochemical Driven Phase Segregation Enabled Dual-Ion Removal Battery Deionization Electrode,” was published in the high-impact international journal Nano Letters.

NaBi3O4Cl2 is the right material with an electrochemically driven phase transformation feature and composed of both Cl– and Na+ ions inside its composition, critical for achieving bifunctional dual-ion storage.
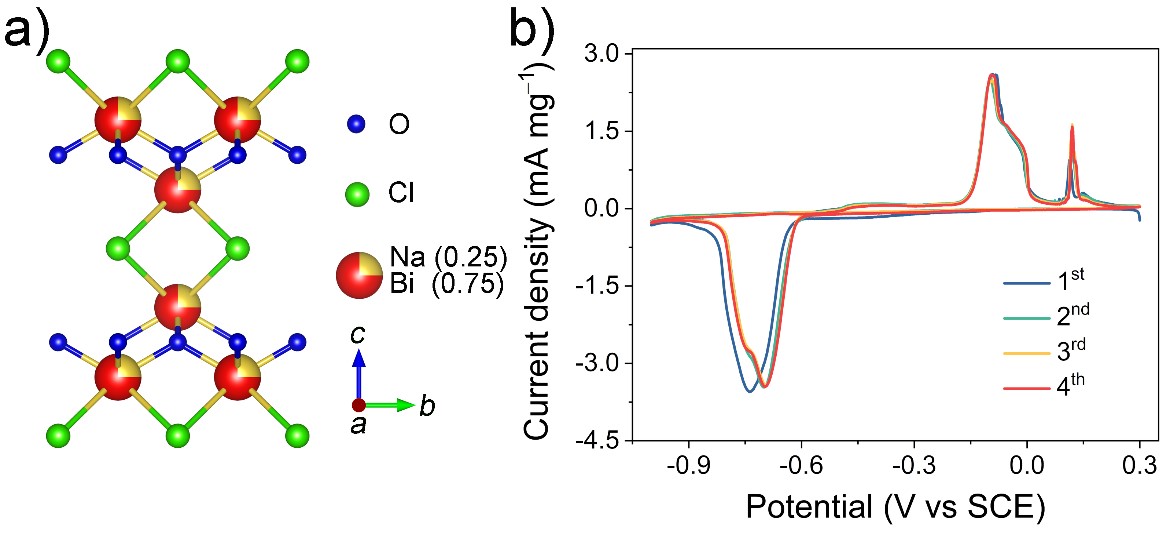
Figure 1. Structure and electrochemical characterization of NaBi3O4Cl2. (a) Crystal structure view along a axis. (b) Cyclic voltammetry (CV) curves in 0.5 M NaCl at a scan rate of 0.2 mV s–1.
With NaBi3O4Cl2, Hong Chen’s team demonstrated the first battery deionization (BDI) electrode, which can simultaneously remove both the Cl– and Na+ with the state-of-the-art high ion removal capacity and excellent cyclic stability. In situ powder X-ray diffraction (PXRD) unravels that the dual-ion removal is attributed to a novel electrochemical-driven phase segregation reaction mechanism. In natural seawater, CV curves, galvanostatic charge/discharge performance, and dual-ion removal capacity suggest NaBi3O4Cl2 is a high-efficiency BDI electrode material for seawater treatment.
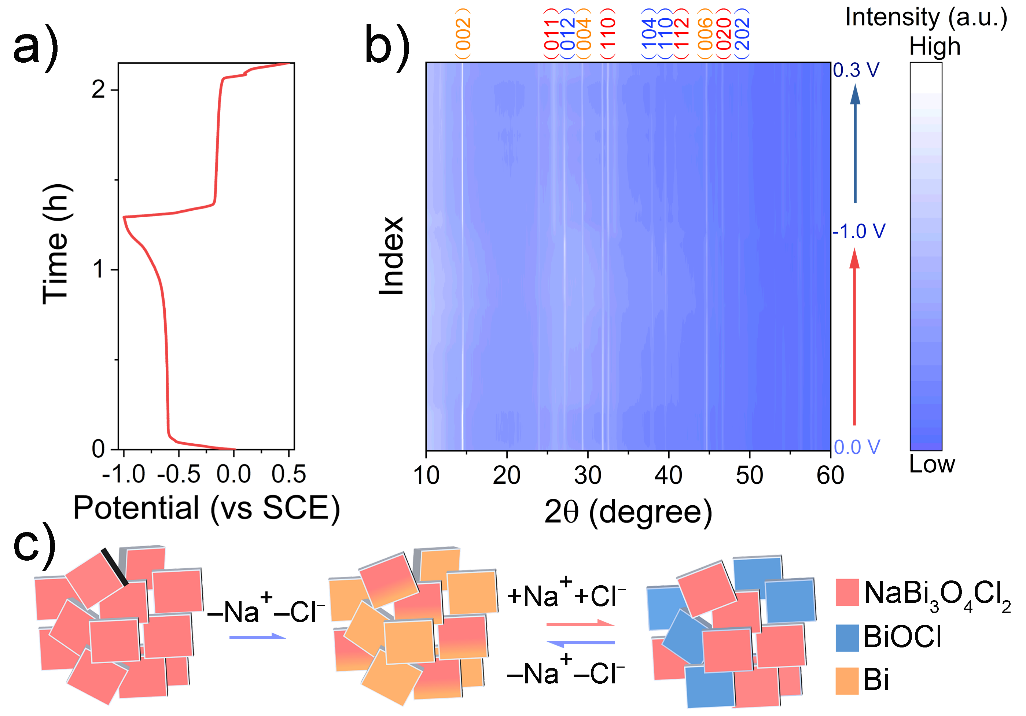
Figure 2. In situ structural evolution under discharge/charge process for NaBi3O4Cl2 electrode. (a) Discharge/charge curve. (b) In situ PXRD patterns. The orange, red and blue diffraction index is attributed to NaBi3O4Cl2, BiOCl, and Bi, respectively. (c) Schematic illustration of first discharge and subsequential reversible electrochemical reaction.
In this work, Hong Chen’s team first demonstrated NaBi3O4Cl2 electrode as an efficient and high capacity dual-ion storage electrode for seawater desalination attributed to its reversible phase segregation reaction under the BDI process. In situ PXRD reveals the novel reversible electrochemical reaction of NaBi3O4Cl2/BiOCl/Bi within this electrode. Due to the fast kinetics and low charge transport resistance, the NaBi3O4Cl2 electrode exhibits the high removal capacity of Cl– and Na+ up to 58.4 mg g-1 and 8.7 mg g-1, respectively, in NaCl solution. Employing the NaBi3O4Cl2 electrode for natural seawater desalination, 80% of its capacity can be retained with good cycling stability and dual-ion removal capacity. The unique dual-ion removal BDI electrode demonstrated with NaBi3O4Cl2 opens a new voyage for the future development of BDI electrode, which paves the way for the energy-efficient BDI technique.
Associate Professor Hong Chen of the School of Environmental Science and Engineering at SUSTech is the corresponding author of this paper. Postdoctoral Wenfei Wei and Ph.D. student Xuezhen Feng are the co-first authors. This work was also supported by Ph.D. student Ranhao Wang and Postdoctorals Renji Zheng and Dazhong Yang.
This work was financially supported by the National Natural Science Foundation of China (NSFC), Natural Science Funds for Distinguished Young Scholars of Guangdong Province, and the Science, Technology and Innovation Commission of Shenzhen Municipality. The transmission electron microscopy, scanning electron microscopy, and PXRD data were collected using equipment from the SUSTech Core Research Facilities.
Paper link: https://pubs.acs.org/doi/10.1021/acs.nanolett.1c01487
Latest News
Related News