True behavior of metal catalysts revealed under ETEM by Chinese researchers
Apr 08, 2020
Ground-breaking research by Chinese scholars has found that metal catalysts behave in a different way than previously believed, thanks to new technology that reveals the behavior of metal catalysts during chemical reactions.
SUSTech Associate Professor Meng Gu (Materials Science and Engineering) and his research team have played a vital role in a collaborative paper that was published in the high-impact academic journal, Nature Catalysis. The paper was titled, “Reversible loss of core-shell structure for Ni–Au bimetallic nanoparticles during CO2 hydrogenation.”
The technology in question is called in-situ environmental transmission electron microscopy (ETEM). It has been used in a wide range of material science research fields, including nanomaterials growth, catalytic reaction, battery reactions, nanomechanics, and high-temperature phase changes. ETEM is different from conventional TEM because ETEM allows scholars to see changes happening in real-time at the atomic scale.
In a catalytic reaction, catalysts are not stationary, with surface atoms influenced by different variables. As a result, the surface atoms tend to move and restructure, so conventional TEM techniques do not allow scientists to assess the reactive surface of a catalyst accurately.
The research group directly observed the change of a nickel-gold (NiAu) bimetal catalyst during the CO2 hydrogenation process by ETEM under real reaction conditions. The proof has revealed the real active surface, providing a new understanding of the catalytic process.
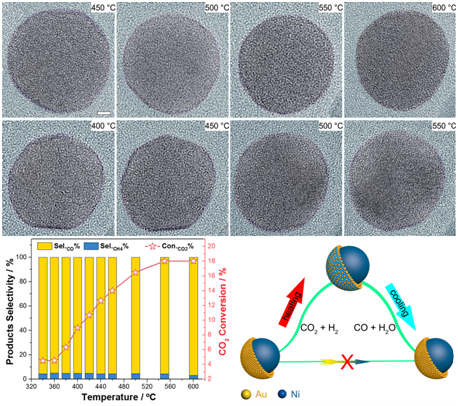
This work reported a NiAu / SiO2 catalyst with a high CO selectivity of 95% and a CH4 selectivity less than 5%, which is different from previous researches that Ni-based catalysts are prone to exhibit a high CH4 selectivity. According to TEM images, this catalyst showed a core-shell structure — a Ni core and 2-3 atomic layers of an ultrathin Au shell, before and after CO2 hydrogenation. Therefore, the high CO selectivity is likely attributed to the ultrathin Au shell.
However, ETEM showed a different active surface. The researchers found that the ETEM images revealed that the core Ni atoms gradually migrated to the surface, forming an alloy with Au. Once the reaction stops, the surface Ni atoms then migrate back into the core to reform the original Ni@Au core-shell structure. The in situ infrared and X-ray absorption spectrums macroscopically confirmed the observations.
The teams combined the observations with their theoretical calculations to propose a new catalytic mechanism. Their work revealed the true active surface of the catalyst, which demonstrates the importance of ETEM, and inspires further research in metal catalysts.
Dr. Xiaoben Zhang (Dalian Institute of Chemical Physics – DICP), Shaobo Han (SUSTech), Beien Zhu (Shanghai Advanced Research Institute), and Guanghui Zhang (Purdue University) were the co-first authors of the paper. Yi Gao (Shanghai Advanced Research Institute), Bing Yang (DICP), Meng Gu (SUSTech), and Wei Liu (DICP) were the co-correspondent authors. Additional contributors came from the University of the Chinese Academy of Sciences, Shanghai Institute of Applied Physics, Dalian University of Technology, and the Université de Strasbourg.
This work received financial support from Talents Innovation Project of Dalian, CAS Youth Innovation Promotion Association and the Natural Science Foundation of China, the Fundamental Research Funds for Central Universities, and the Key Research Program of Frontier Sciences, CAS.
Paper link: https://www.nature.com/articles/s41929-020-0440-2
Latest News
Related News