Transparent models of organs to deliver better health care
Apr 14, 2020
The rapid development of biotechnology has seen the dramatic increase in applications for transparent models of organs for the observation and study of the delicate three-dimensional structure of organs and mechanisms of diseases. An international collaboration led by the Southern University of Science and Technology (SUSTech) has made significant progress in the construction of transparent liver organs modeling liver cancer interventional treatments, providing significant help to researchers, doctors, and patients.
Assistant Professor Qiongyu Guo of Biomedical Engineering at the SUSTech led her research team to work with the National University of Singapore and Henan University to publish a paper in the high-impact academic journal, Biomaterials (IF = 10.273). The article was titled “Decellularized liver as a translucent ex vivo model for vascular embolization evaluation.”
Approximately 850,000 new cases of liver cancer are reported worldwide annually. Liver cancer has placed a heavy burden on society in many countries and is currently the leading cause of death for men under 50 years of age. Hepatocellular carcinoma (HCC), which accounts for 85%–90% of primary liver cancers, is the predominant pathological type of malignant liver tumors.
Transcatheter arterial chemoembolization (TACE), which applies embolic agents to selectively occlude tumor-supplying hepatic arteries, is currently the mainstay treatment for patients who have lost the opportunity for resection surgery. However, there is not an adequate model to evaluate embolization performance for TACE treatment, which has affected the development of new embolotherapies.
In vitro models such as microfluidics have been used to evaluate the performance of these agents. However, the materials used in the models do not correctly replicate the mechanical properties of blood vessels. The model channels are often too simple to simulate the complexities of HCC. The limited spatial resolution of X-ray-based instruments available for TAE/TACE and the lack of imageability of most solid embolic agents themselves prevent the accurate study of the penetration depth and embolization endpoints in animal models. Thus, the development of a new TACE model system that accurately evaluates embolic agents is vital for this clinical field.

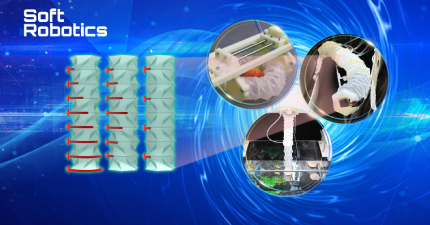
Figure 1. Quantitative analysis of the vascular systems of a translucent liver model
The research group has proposed a new strategy for assessing vascular embolization by using decellularized whole livers as a clearing in vitro model. In recent years, decellularization has been used primarily for regenerating organs. The team developed a transparent liver by applying a strictly controlled decellularization perfusion method. They completely removed the cells while maintaining the extracellular matrix and the vascular system within the liver. The model of the liver was translucent, allowing the vascular system to be viewed through a variety of imaging tools (Figure 1).

Figure 2. Evaluation of different embolic agents in a cleared, isolated liver model
The researchers successfully used the translucent model to evaluate different types of embolic agents (Figure 2). They observed that the embolization endpoint of a liquid embolic agent depends strongly on the injection pressure and the location of the injection. Solid embolic agents tend to have a reduced density near the end of an embolization site. These findings confirm that particle size and penetration depth are two key factors that determine embolic distribution.

Figure 3. Dynamic monitoring of embolization kinetics of liquid embolic agent iodized oil
The research team also examined the embolization kinetics of TACE treatment, and for the first time, evaluated the correlation between the embolization pressure and the penetration depth as well as the liver morphologies in the decellularized liver model (Figure 3). This model enables the monitoring of the spatiotemporal location of embolic agents. The finding is critical for real-time analyses of the effectiveness of embolization formulations for TACE treatment.
This research opens up new methods for developing transparent organ models for visualization research and evaluation of clinical treatment methods. It will provide more effective assessment strategies for the translational research of various biotechnologies and biomaterials.
SUSTech research assistant Yanan Gao is the first author of the paper with research assistant Zhihua Li has made vital contributions to the paper. Assistant Professor Qiongyu Guo is the corresponding author of the article, and SUSTech is the first communication unit. Additional contributions came from the National University of Singapore (Department of Biomedical Engineering, Yong Loo Lin School of Medicine, and Mechanobiology Institute), the First Affiliated Hospital of SUSTech (Shenzhen People’s Hospital), SUSTech (Materials Science and Engineering, Academy of Advanced Interdisciplinary Studies), Henan University (College of Medicine), A*STAR (Institute of Bioengineering and Nanotechnology), Singapore-MIT Alliance for Research and Technology (CAMP), and Southern Medical University (Gastroenterology Department).
This research received support from the Key-Area Research and Development Program of Guangdong Province, National Natural Science Foundation of China, the startup funding from SUSTech, and the SMART CAMP and Mechanobiology Institute of Singapore funding.
Paper link: https://www.sciencedirect.com/science/article/pii/S0142961220301010
Latest News
Related News