Energy solutions advanced through cutting-edge research in Shenzhen
Apr 02, 2020
With the world grappling with a wide range of issues around energy supply and use, a group of interdisciplinary scholars from Southern University of Science and Technology (SUSTech) has made new findings in three distinct areas of material science.
Chair Professor Hsing-Lin Wang (Materials Science and Engineering) has led his research group to publish cutting-edge papers in high-impact academic journals, Nano Energy (IF = 15.548), ACS Nano (IF = 13.903), and Journal of the American Chemical Society (IF = 14.695), also known as JACS.
The paper published in Nano Energy was titled, “Single Fe atoms anchored by short-range ordered nanographene boost oxygen reduction reaction in acidic media.” The article sought to develop efficient and stable non-platinum-based (Pt) electrocatalysts.
These electrocatalysts play a vital role in clean energy conversion devices, and generating them at a commercial scale is of critical importance. The primary focus of the paper was to find an alternative to the scarce and expensive Pt, ideally using a metal that is cheap and abundant throughout the world.
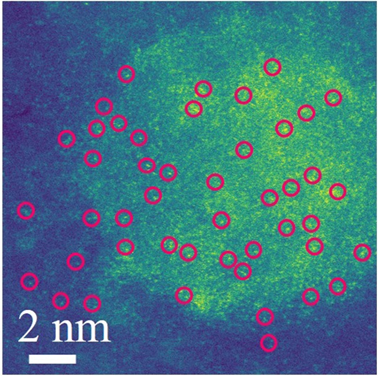
Figure 1. The Cs-TEM image of the single-atom catalysts for oxygen reduced reaction in acidic media.
The research team synthesized single iron (Fe) atoms within a nitrogen-doped nanographene (Fe1-N-NG). It was loaded on to a reduced graphene oxide (RGO) structure to create a highly efficient electrocatalyst with a well-defined structure. The use of this architecture, known as nanographene, allowed the scholars to precisely control its atomic structure during organic synthesis.
The researchers found that the half-wave potential of the Fe1-N-NG/RGO electrocatalyst was 0.84 V, just 20 mV (2.38%) of commercial Pt/C catalysts. Their newly-created electrocatalyst was highly stable, with the half-wave potential changing by no more than 5 mV over fifteen thousand cycles.
Their findings offer significant opportunities for further research into proton exchange membrane fuel cells (PEMFCs) as a sustainable energy conversion technology.
The co-first authors of the paper are SUSTech postdoctoral researcher Shaoqing Chen and Nianji Zhang. The research team of Chair Professor Hsing-lin Wang collaborated with Associate Professor Hu Xu (SUSTech Department of Physics), whose team created a theoretical model for simulating the electrocatalytic reaction process.
Additional support came from the Los Alamos National Laboratory, the State Key Lab of Inorganic Synthesis and Preparative Chemistry (Jilin University), the Hefei National Laboratory for Physical Sciences at the Microscale (University of Science and Technology of China), and the State University of New York (Buffalo).
The research received funding support from the Leading Talents of Guangdong Province Program, the Shenzhen Basic Research Fund, and the National Key Research and Development Program of China.
Article link: https://www.sciencedirect.com/science/article/abs/pii/S2211285519308717
The paper published in ACS Nano was titled, “C60(OH)12 and Its Nanocomposite for High-Performance Lithium Storage.” This article examined the use of organic carbon materials for improving lithium-ion batteries.
The use of rechargeable lithium-ion batteries (LiBs), as an energy storage device, has been widely used in technologies, ranging from portable electronics to electric vehicles
The energy densities of current commercialized batteries do not meet the demands of consumers. Enhancing the capacity of the anode in the LIBs by reducing the weight of anode electrodes is one alternative to improving the energy density of batteries.
The majority of research in this field had previously focused on inorganic materials, which lack the long-term capacity during charging and discharging. With that in mind, the researchers used C60(OH)12 and graphene oxide (GO) as the raw materials to create a high-performance anode nanocomposite for lithium storage.
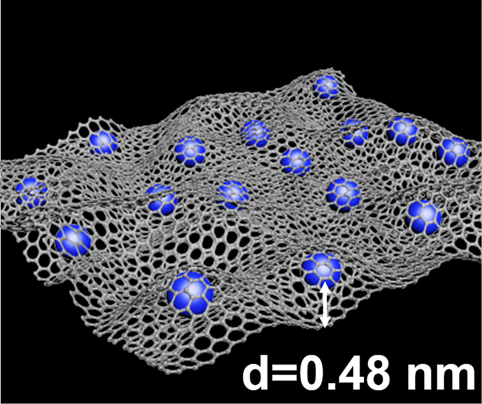
Figure 2. High-performance lithium storage materials based on C60 Derivative and graphene oxide composite.
The research team was able to show a significantly higher reversible cycling capacity than either C60(OH)12 and GO alone. The improved electrochemical performance can be attributed to the distribution of C60(OH)12 between GO layers. The electrochemical performance is also improved through the rapid cycling of lithium ions (Li+), and the larger number of oxygen groups also support improved performance.
The co-first authors of the paper are SUSTech graduate student Zhengang Li and National Taiwan University (NTU) Dr. Shih-Hao Wang. The correspondent authors were SUSTech Hsing-Lin Wang and NTU Professor Leeyih Wang. Other supporting institutions were the Harbin Institute of Technology and Northeast Normal University.
The teams received support from the Leading Talents of Guangdong Province Program, the Key-Area Research and Development Program of Guangdong Province, the Shenzhen Basic Research Fund, the National Key Research and Development Fund of China, the Guangdong Provincial Key Laboratory of Energy Materials for Electric Power, and the Shenzhen Key Laboratory Project. The team sincerely thanks the Center of Atomic Initiative for New Materials (NTU) and the Ministry of Science and Technology (MOST) for their financial support, as well as the Instrument Center at NTU for their technical support.
Article link: https://pubs.acs.org/doi/10.1021/acsnano.9b06791
The paper published in JACS was titled, “Evidence of Ferroelectricity of All-Inorganic Perovskite CsPbBr3 Quantum Dots.” Quantum dots offer enormous potential across a vast array of fields, but little research has been conducted into their ferroelectric properties. Ferroelectricity is a physical property of certain materials where they show an electric polarization depending on the application of an external electric field.
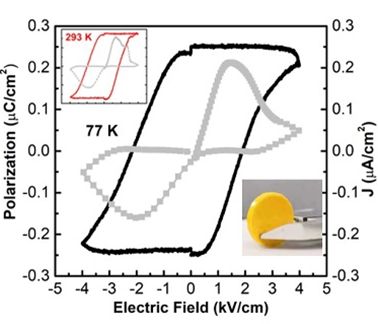
Figure 3. Ferroelectric properties of all inorganic halide perovskite CsPbBr3 quantum dots
The researchers discovered, for the first time, that an inorganic halide perovskite made up of cesium, lead, and bromine (CsPbBr3). They synthesized it into a quantum dot and were able to show a reversible phase change between 263 K and 298 K. Their further testing showed that they could combine the ferroelectric and optoelectric properties in quantum dots for further interdisciplinary research. It opens up new horizons to explore high-performing multifunctional materials.
The first author of the paper is doctoral candidate Xia Li. The correspondent authors are SUSTech Chair Professor Hsing-Lin Wang and Jilin University Professor Hongming Yuan. Other contributing researchers were from Southern University of Science and Technology (SUSTech), State Key Lab of Inorganic Synthesis and Preparative Chemistry (Jilin University), the Institute of High Energy Physics, and the Spallation Neutron Source Science Center.
Article link: https://pubs.acs.org/doi/abs/10.1021/jacs.9b12254
Latest News
Related News