Improved stability and duration from next-generation high-energy batteries
Apr 02, 2020
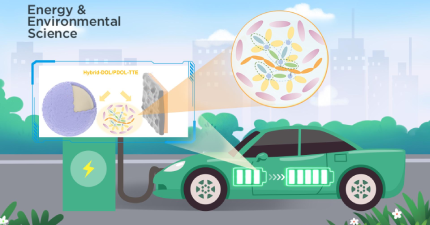
With lithium-sulfur batteries (LiSBs) becoming the focus of increasing research, a research team at Southern University of Science and Technology (SUSTech) has found a new direction in the electrolytes that could lead to their improved performance and stability.
Associate Professor Yonghong Deng (Materials Science and Engineering) has led her research team to publish their latest research progress in new lithium salts in the high-impact academic journal, Advanced Energy Materials (IF = 24.88). Their paper was titled, “New Lithium Salt Forms Interphases Suppressing Both Li Dendrite and Polysulfide Shuttling.” Nano Letters (IF = 12.7) published a second paper, under the title, “Lithophilic Zn Sites in Porous CuZn Alloy Induced Uniform Li Nucleation and Dendrite-free Metal Deposition.”
The first paper was published in Advanced Energy Materials, under the title, “New Lithium Salt Forms Interphases Suppressing Both Li Dendrite and Polysulfide Shuttling.”
LiSBs offer enormous promise as high-specific energy batteries, primarily due to their high energy density. However, LiSBs experience significant problems around the anode and cathode that hinder their potential for commercial applications. Scholars have studied new electrolyte additives and solvents, as well as using unsaturated or Li salts containing fluorine (Fl) to solve the problems. The larger challenge has been around the design and synthesis of Li-salt anions, which has lacked significant scientific research. There are many physical and chemical properties associated with Li-salt anions that mean that they have not been effective thus far, so finding a Li-salt that can form a stable electrolyte around both electrodes is vital.
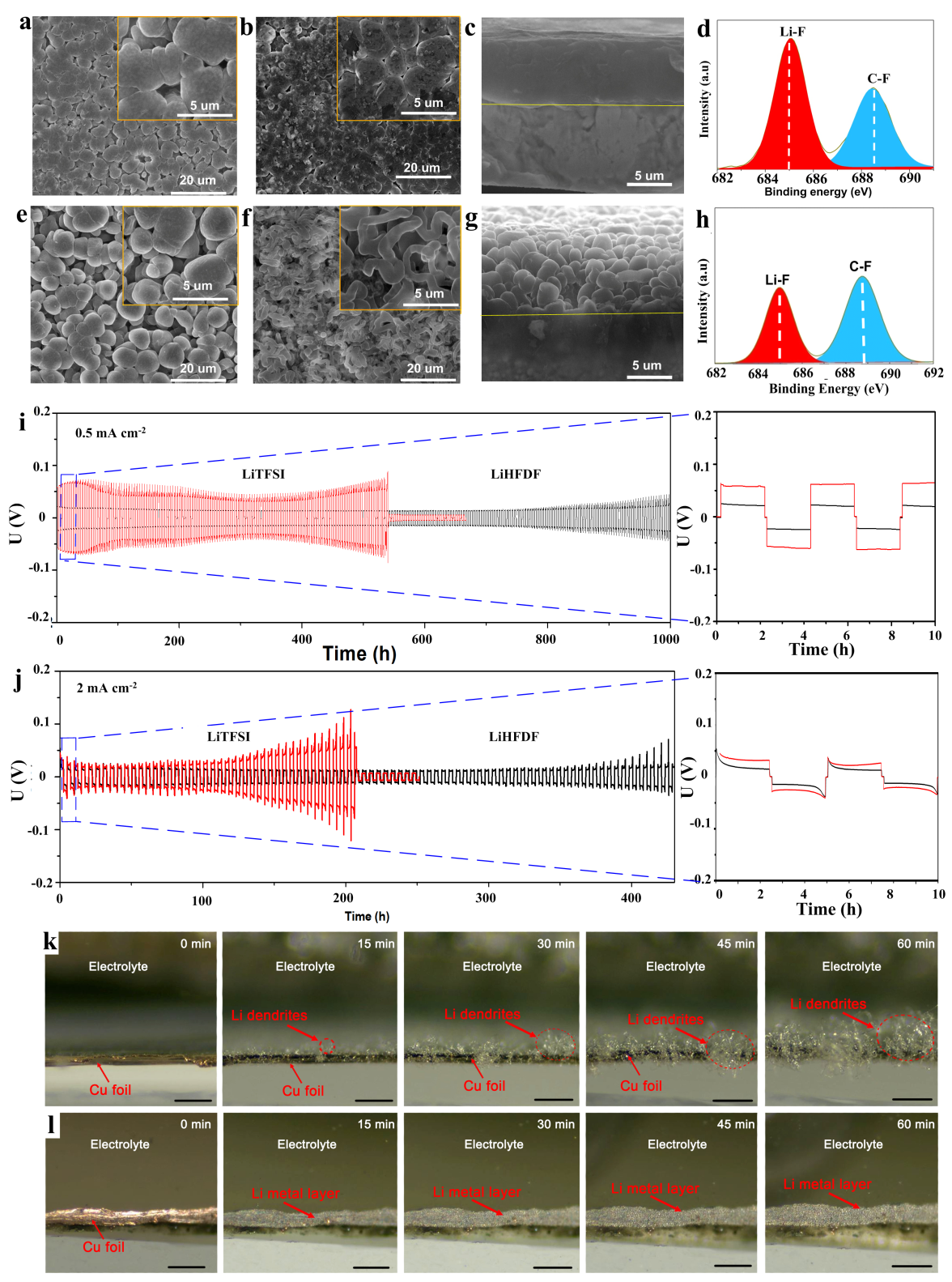
The research team developed a new unsaturated Li-salt (LiHFDF) that would decompose on the surface of positive and negative electrodes to form a LiF-rich electrolyte environment during charge-discharge cycles. It suppressed the growth of Li-dendrites and the shuttle effect of Li2Sn.
Their findings showed a better LiF-rich electrolyte environment around the electrodes, far more than found under solvents and additives. Their testing under a transmission electron microscope (TEM) indicated that their LiHFDF Li-salt would form a CEI film on the surface of both electrodes during different stages of the charge-discharge cycle. They were also able to show that their scanning electron microscope (SEM) testing that the lithium plating that would occur was dense and plat, preventing significant lithium dendrite growth.
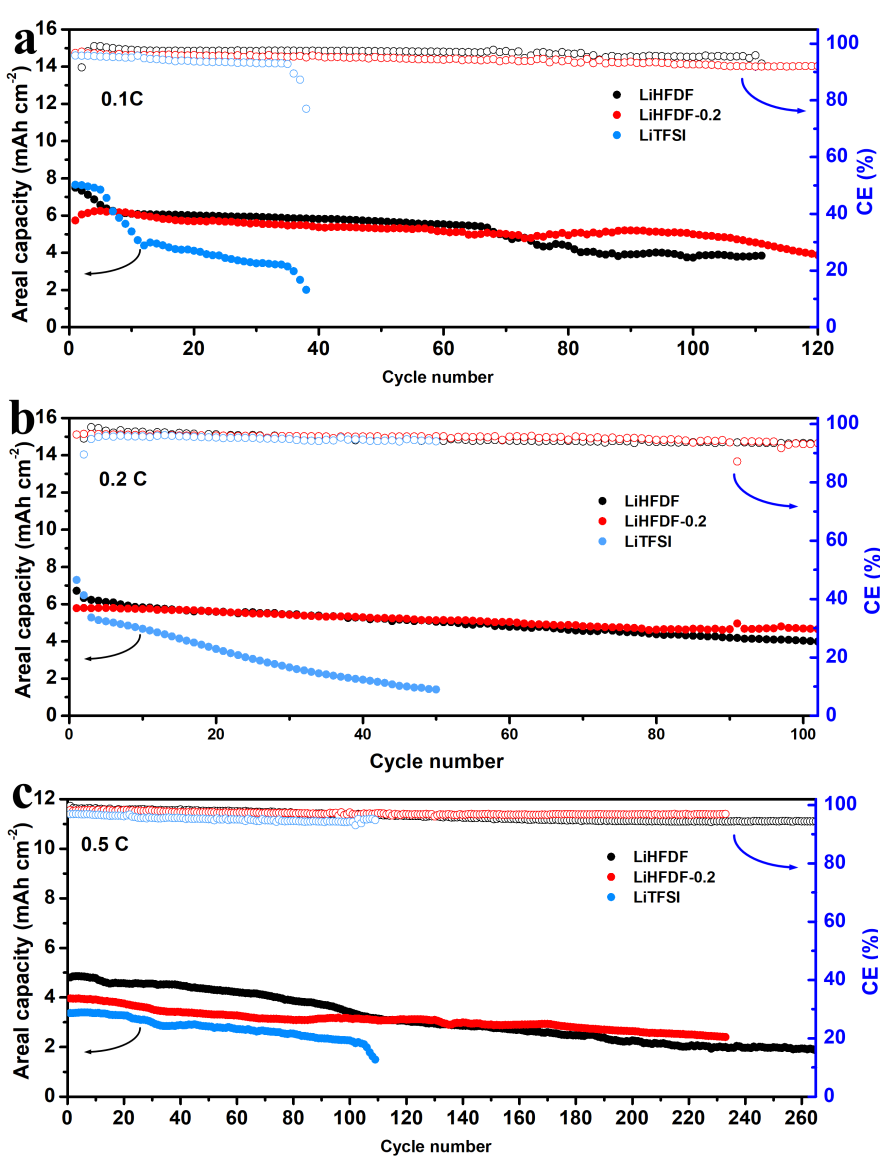
Further testing also showed improved charge-discharge cycle stability and reduced battery polarization, which means increased stability from the LiHFDF and reduced resistance. The LiSBs with LiHFDF and these new batteries were shown to have significantly improved cycle stability and coulomb efficiency (proportion of electrons supplied which are recovered from storage), even as an additive.
This research has developed a new Li-salt that can provide a more stable and efficient lithium-sulfur battery. Such a development can be applied for many lithium-ion battery systems around the world.
The co-first authors were SUSTech-Peking University join-trained doctoral candidates Yingling Xiao and Bing Han. SUSTech Associate Professor Yonghong Deng and US Army Research Laboratory Dr. Kang Xu are the co-correspondent authors, with SUSTech as the first communication unit. Additional contributing units were the School of Advanced Materials at Peking University (PKU) and the Institute of Textile and Clothing (IKTC) at the Hong Kong Polytechnic University (PolyU). The research received support from the R&D Projects in Key Areas of Guangdong Province.
Paper link: https://onlinelibrary.wiley.com/doi/10.1002/aenm.201903937
The paper, “Lithophilic Zn Sites in Porous CuZn Alloy Induced Uniform Li Nucleation and Dendrite-free Metal Deposition,” published in Nano Letters examined new techniques for minimizing lithium (Li) dendrites. It also sought to regulate the infinite changes in volume lithium undergoes during long-term repetitive stripping and plating cycles. These problems occur during charge and discharge cycles, so being able to find a solution to these problems would improve the effective use of Li-salts in high-energy batteries.
A lot of research has been done on the use of three-dimensional (3D) lithiophilic hosts, as they are the most effective ways to regulate Li dendrites and volume change in the working Li metal anodes. In high-temperature environments, these 3D lithiophilic hosts melt or lose their adhesive bonds under the thermal infusion method. Thus, finding a 3D lithiophilic host that can handle a high-temperature environment while exploring an internal mechanism for homogenous Li deposition is highly desirable to creating Li metal anodes.
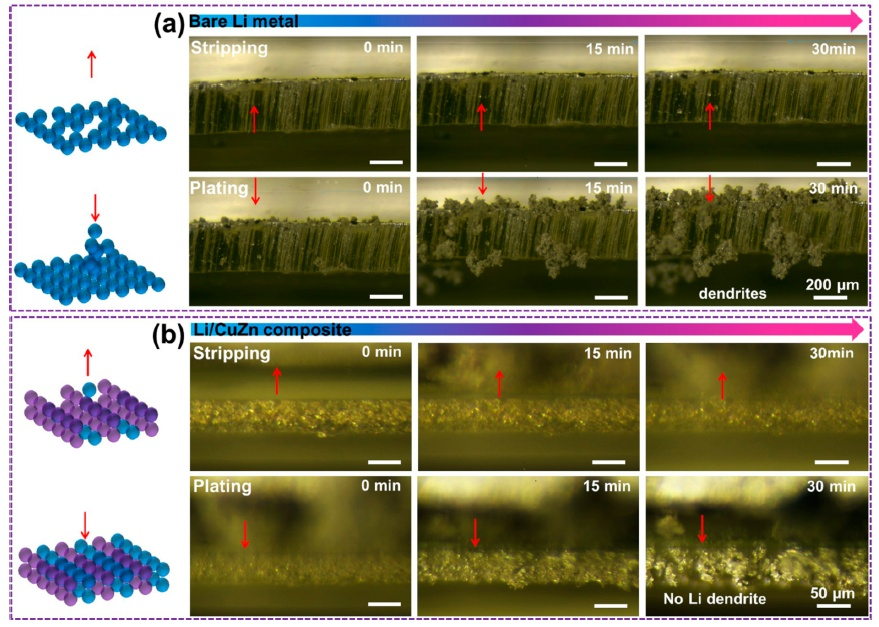
The research team used a copper-zinc (CuZn) alloy in a 3D porous host to contain anchored lithiophilic Zn sites. This new approach ensures that the host can handle a thermal melting high-temperature environment under the thermal infusion strategy. It dispersed the Li nucleation sites, which encouraged the uniform deposition of Li through the stripping/plating cycle.
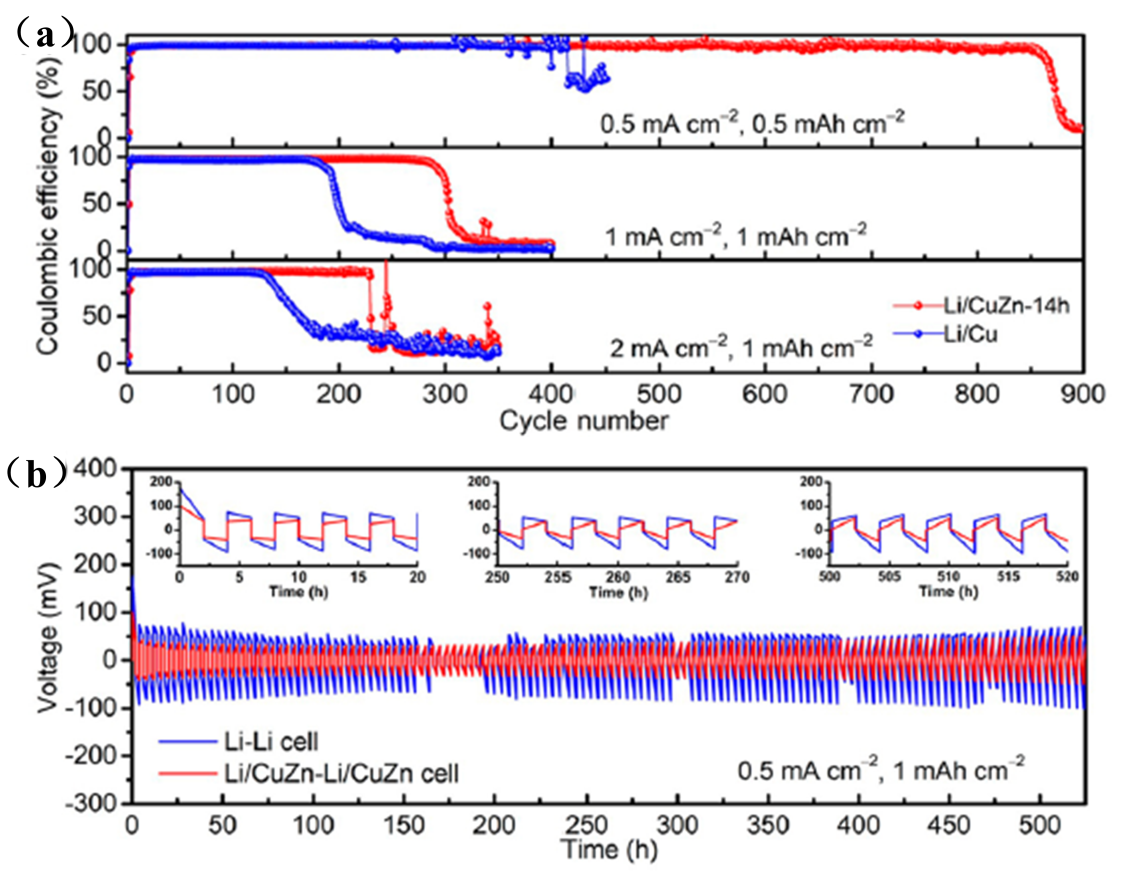
On testing the new host, the researchers found that the Li host sites absorb more than previous examples, based on the analysis of nucleation overpotential, binding energy calculation and optical observation of Li plating/stripping cycles. It shows that Li nucleation and dendrite-free Li deposition can occur within the host while significantly improving its electrochemical performance.
The co-first authors were SUSTech postdoctoral researcher Shang-Sen Chi and research assistant Qingrong Wang. The co-correspondent authors were Hefei National Laboratory for Physical Sciences at the Microscale (University of Science and Technology of China) Professor Yan Yu and SUSTech Associate Professor Yonghong Deng. Additional contributors came from the Academy for Advanced Disciplinary Studies (AAIS) at SUSTech, the Shenzhen Key Laboratory of Solid-State Batteries, the Guangdong Provincial Key Laboratory of Energy Materials for Electric Power, the Research Institute of Materials Science at South China University of Technology (SCUT), Dalian National Laboratory for Clean Energy (DNL) at the Chinese Academy of Sciences (CAS), and the State Key Laboratory of Fire Science (SKLFS) at USTC.
This research received support from the National Key R&D Program of China, R&D Project in Key Areas of Guangdong Province, the National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities, Dalian National Laboratory For Clean Energy (DNL) Cooperation Fund, the CAS, Shenzhen Key Laboratory of Solid-State Batteries, Guangdong Provincial Key Laboratory of Energy Materials for Electric Power, and the China Postdoctoral Science Foundation.
Article link: https://pubs.acs.org/doi/10.1021/acs.nanolett.0c00352
Latest News
Related News