Development of improved electrocatalysts progresses under SUSTech research
Sep 14, 2019
Ammonia is an ideal carbon-free energy storage material owing to its high energy density (4.32 kWh/L), high weight fraction of hydrogen (17.65%), ease of liquefaction under mild conditions, and inexpensive transportation. Industrially, ammonia synthesis is realized by the energy intensive Haber-Bosch process, where nitrogen and hydrogen are reacted at high temperatures (~450 °C) and pressures (10,000 kPa) over an iron-based catalyst.
Research led by Chair Professors Hui Li and Haijiang Wang of Southern University of Science and Technology (SUSTech), in conjunction with Professor Minhua Shao of the Hong Kong University of Science and Technology (HKUST) has seen several papers published by Ph.D. student Yao Yao. The papers, published in the Journal of the American Chemical Society, ACS Energy Letter, Small Methods, and Electrochemical Energy Review, substantially develop the field’s understanding of the electrochemical synthesis of ammonia
As hydrogen used in the Haber-Bosch process is mainly produced by the steam-reforming process, CO2 emission in this process is another significant environmental issue. In 2015, the energy consumption and CO2 emission during the Haber–Bosch process accounted for 2% and 0.5% of the world annual total. As such, a carbon free and energy efficient process for ammonia production is highly demanded. In this regard, the synthesis of ammonia from N2 with renewable electricity has garnered great interest over the past several years.
The electrochemical process for ammonia synthesis enables the energy contained in renewable energy could be directly converted into chemical energy, thus streamlining the procedures and enhancing the process efficiency. In contradistinction to the Haber-Bosch process, the electrochemical synthesis route is a much more environmentally friendly alternative operating under mild conditions with zero carbon dioxide (CO2) emissions. Ammonia could be synthesized with the nitrogen and water as the feedstock under driving force of electricity.
However, the cathodic reaction, that is the nitrogen reduction reaction (NRR), has a theoretical potential of 0.06 V under ambient conditions, which is very close to that of the hydrogen evolution reaction (HER). Due to much slower reaction kinetics of NRR in contrast to HER on most catalyst surfaces, and very low nitrogen concentration in the electrolyte, the development of NRR has been greatly limited. Although a large amount of effort has been putted into ammonia electrochemical synthesis, notably the NRR electrocatalysts, there is still an obvious gap between the current status and the application of the NRR research, because there is a lack of deep understanding on the NRR reaction mechanism.
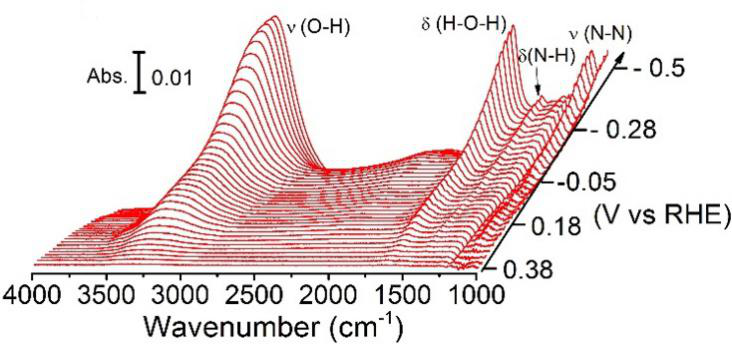
Despite that understanding the NRR mechanism is vital for the rational design of more advanced electrocatalysts, experimental studies in this area are rather rare due to the significant influence of HER. In their research, surface enhanced infrared absorption spectroscopy (SEIRAS) was employed to study the reaction mechanisms of nitrogen reduction on an Au thin film for the first time. (Figure 1) During the nitrogen reduction, the N2Hy species was detected with bands at 1453 cm-1 (H-N-H bending), 1298 cm-1 (-NH2 wagging), and 1109 cm-1 (N-N stretching) at potentials below 0 V against reversible hydrogen electrode. This result indicates that the nitrogen reduction reaction on Au surfaces follows an associative mechanism, and the N≡N bond in N2 tends to break simultaneously with the hydrogen addition. By comparison, no absorption band associated with N was observed on Pt surfaces under the same condition. This result is consistent with the low efficiency of nitrogen reduction on Pt due to the much faster kinetics of hydrogen evolution reaction. The related results were published on Journal of the American Chemical Society. (J. Am. Chem. Soc. 2018, 140, 1496-1501) and entitled “A Spectroscopic Study on the Nitrogen Electrochemical Reduction Reaction on Gold and Platinum Surfaces.”
Original paper – https://pubs.acs.org/doi/10.1021/jacs.7b12101
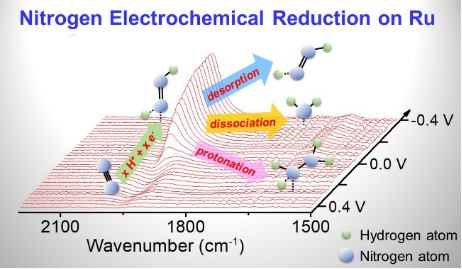
On Ru surface, the *N2Hx (0≤x≤2) was detected with the band of N=N stretching (~1940 cm−1) at potentials below 0.2 V in a N2-satureated HClO4 solution. (Figure 2) It indicated that the *N2Hx could be further reduced to *N2Hy (3≤y≤4), or be dissociated into *NH2. In addition, it also could be desorbed from the Ru surface. Since it extremely unstable and even more active than hydrogen, it could be decomposed into ammonia and nitrogen in the electrolyte after it desorbed from the electrode. It may be the reason why ammonia could be synthesized on Ru-based electrocatalysts with low overpotentials. Moreover, they also found that the nitrogen adsorption strength on Ru surface is related to the pH of the electrolyte. In acidic electrolyte, the nitrogen molecules tend to adsorb on Ru surface; while in alkaline electrolyte, no chemical adsorbed nitrogen was detected on Ru surface. It gives the reason why Ru-based electrocatalysts exhibited best performance in acidic electrolyte rather than the alkaline one in recently published papers. This work is recently published on ACS Energy Letter, and becomes the “Most Read Articles for previous 30 days” of this journal. (ACS Energy Lett. 2019, 1336-1341.) The paper was titled “Electrochemical Nitrogen Reduction Reaction on Ruthenium.”
Original article – https://pubs.acs.org/doi/10.1021/acsenergylett.9b00699
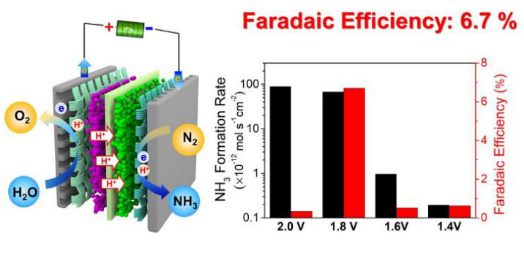
Additionally, they found that the chromium oxynitride (CrO0.66N0.56) nanoparticles has excellent NRR performance in an home-made proton exchange membrane electrolyzer under ambient conditions. The highest ammonia formation rate of 8.9×10-11 mol s-1 cm-2 and 15.56 μg h-1 mg-1cat were achieved at a cell voltage of 2.0 V. The highest Faradaic efficiency of 6.7% was obtained at a cell voltage of 1.8 V. (Figure 3) Their findings demonstrate that metal nitride-based materials could be promising electrocatalysts toward NRR and could guide rational design of more advanced catalysts for various reactions. The related research was published on Small Methods, entitled “Chromium Oxynitride Electrocatalysts for Electrochemical Synthesis of Ammonica under Ambient Conditions,“ (Small Methods, 2018, 1800324.) An invited NRR review of them was recently accepted by Electrochemical Energy Reviews, and is due for publication later this year.
Small Methods Paper –https://onlinelibrary.wiley.com/toc/23669608/2019/3/6
Yao Yao is a joint Ph.D. student between the Department of Materials Science and Engineering (MSE) at SUSTech and Hong Kong University of Science and Technology (HKUST) in 2016, under the supervision of Prof. Hui Li (MSE Chair Professor), Prof. Haijiang Wang (Chair Professor of the Department of Mechanical and Energy Engineering), and Prof. Minhua Shao (Professor in HKUST). Her research is focused on the electrochemical nitrogen reduction reaction mechanism study and electrocatalysts design.
The research is supported by Shenzhen Peacock Plan, Development and Reform Commission of Shenzhen Municipality, Guangdong Innovative and Entrepreneurial Research Team Program, Guangdong Provincial Key Laboratory of Energy Materials for Electric Power.
Latest News
Related News